A Novel Prognosis Model based on Comprehensive Analysis of Pyroptosis-Related Genes in Breast Cancer
Cell Death
Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, or may result from such factors as disease, localized injury, or the death of the organism of which the cells are part. Apoptosis or Type I cell-death, and autophagy or Type II cell-death are both forms of programmed cell death, while necrosis is a non-physiological process that occurs as a result of infection or injury.
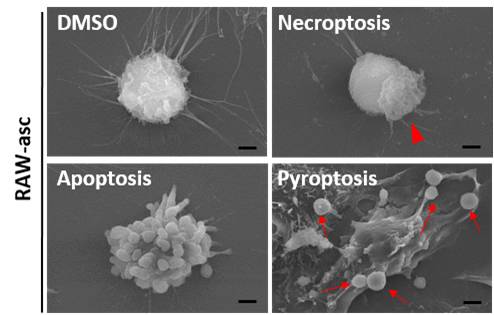
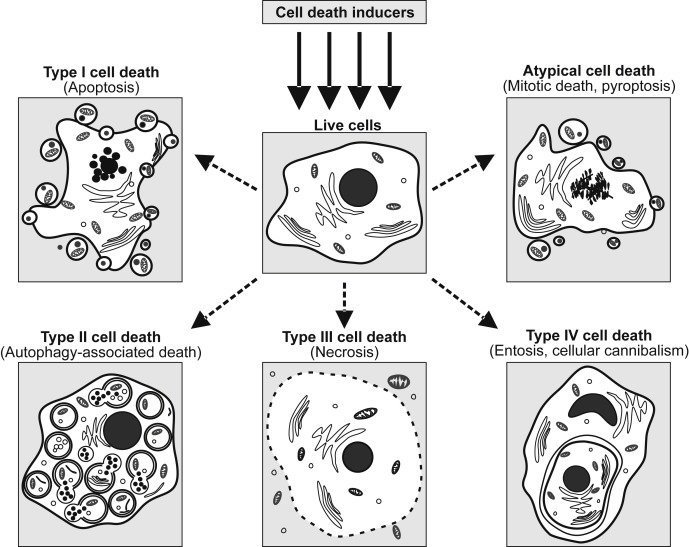
Pyroptosis
Pyroptotic cells generate a low, positive signal when analyzed with a TUNEL assay since the DNA fragmentation is random and the nucleus stays intact. However, other assays are required to differentiate between pyroptosis, other types of necrosis, and apoptosis.
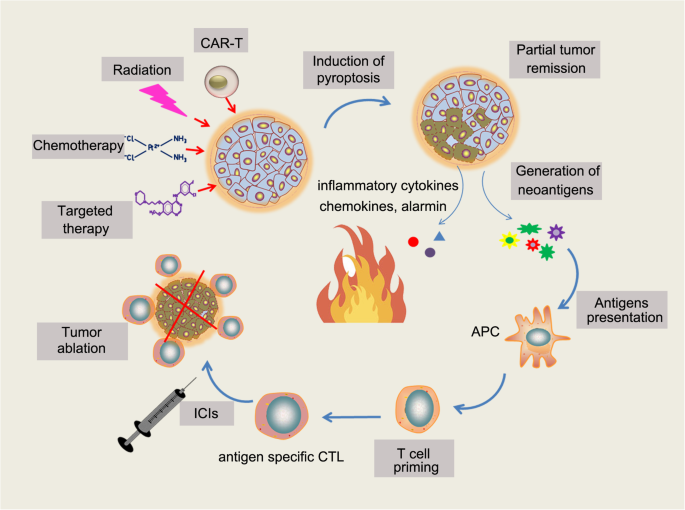
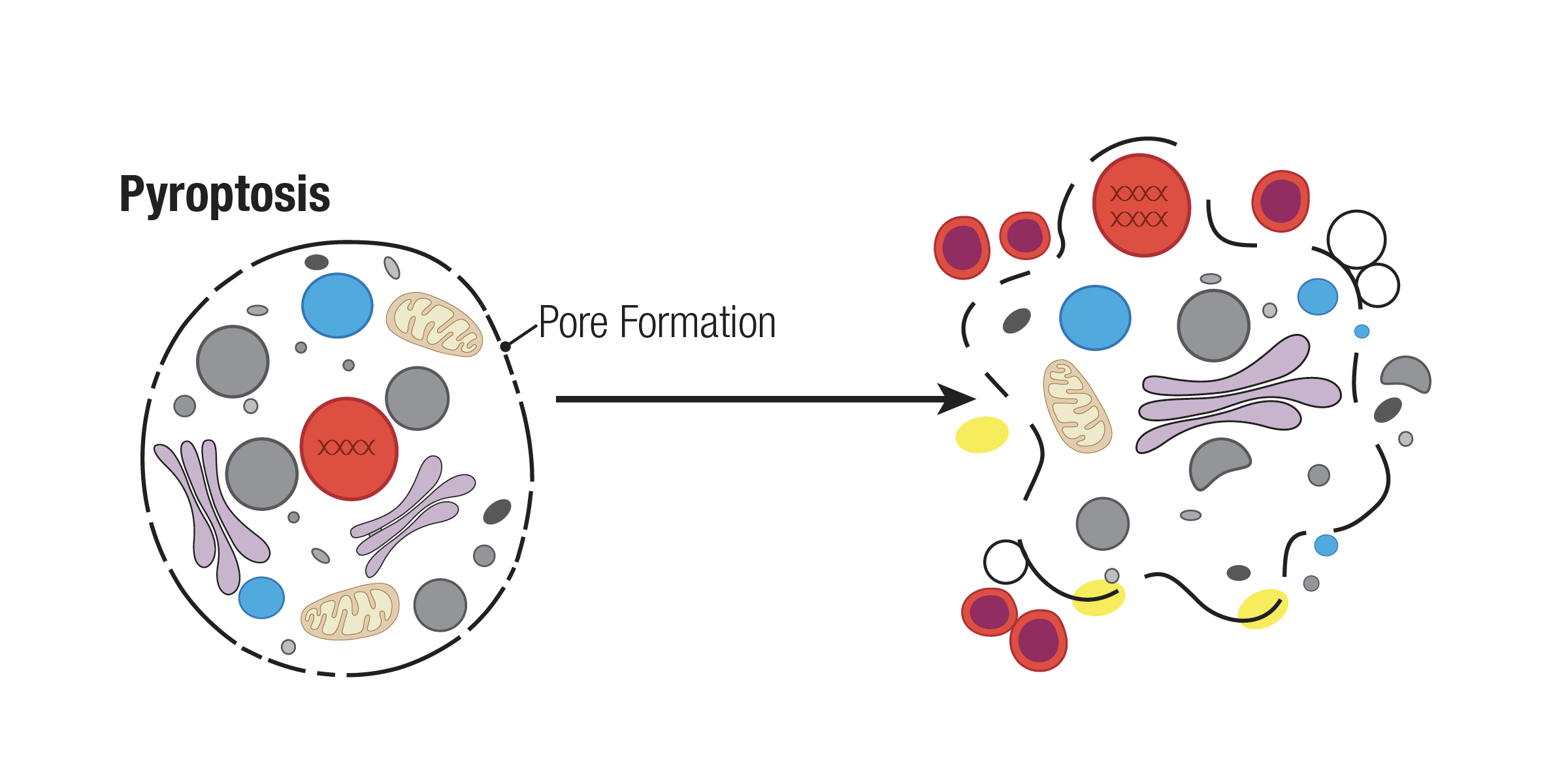
Pyroptosis is mainly characterized by the N-terminal cleavage of gasdermin D (GSDMD), which results in its oligomerization to form a lytic pore in the plasma membrane. This cleavage process relies on the activity of inflammatory caspases 1, 4, 5, and 11, which are different from the caspases that are active during apoptosis. Thus, monitoring cleavage of GSDMD and its related family members, as well these inflammatory caspases, is key to studying pyroptosis.
The inflammatory caspases play an important role in the innate immune system and enable immune cell response to bacterial infection, as they are involved in processing and secreting pro-inflammatory cytokines, such as pro-ILβ and pro-IL18.
Background and Significance
In this work, the related genes of pyroptosis in breast cancer were analyzed, and a new prognosis model was proposed, which can predict the risk of patients. As one of the most common female diseases, breast cancer remains a global challenge, which has caused over 685,000 deaths in 2020. Although current comprehensive treatments for BC have significantly improved the survival rate of patients, the prognosis is not given much attention. Besides, Modern studies demonstrate that there’s a complex relationship between pyroptosis and cancer. However, the study of cell pyroptosis in Breast cancer is still limited. Therefore, establishing a good prognostic model based on pyroptosis-related genes to predict a patient's risk and guide the clinic is significant.
Methods and Results
Our study includes the following parts. 1) By the Wilcoxon Test, we screened 26 pyroptosis-related genes and analyzed their association with different figures. Furthermore, by univariate Cox regression, we obtained 3 pyroptosis-related prognostic genes, which we believe that these genes are significantly different between normal and BC patients. 2) To explore the subtypes of breast cancer, not only the Consensus Clustering was performed, but also two other techniques for classification verification. 3) The BC prognostic model was constructed to distinguish the degree of disease in patients, the TCGA and GEO cohorts have verified the effect of the model on ROC curve, survival curve, risk analysis and PCA demonstration, respectively. 4) Biological function analysis and immune microenvironment analysis were carried out, such as GO and KEGG enrichment analysis and ssGSEA analysis.
Novelty
1) Inspired by machine learning, the EM and Xmeans machine learning algorithms were applied as the verification and comparison of BC subtype classification effect, which has more real and objective evaluation results. (Table 2 of the Manuscript) This led to a new way of clustering patients for researchers in the field of bioinformatics, which can be compared with Consistent clustering. 2) The Random Forest algorithm was applied to construct the prognosis model and achieved good results. The future prognostic model may provide guidance for clinicians and explore ways to treat breast cancer from the perspective of pyroptosis. To learn more, please click here for more information.